A newfound immature microorganism that keeps bones developing
The development of long bones in youthful vertebrates (counting kids) is driven by the development plate, a zone of ligament that isolates each finish of the bone from the primary shaft. The development plate contains three unmistakable kinds of ligament cell (chondrocyte). Round chondrocytes in the resting zone of the development plate separate into level, multiplying chondrocytes that structure sections along the bone's longitudinal hub. These cells later progress toward becoming hypertrophic chondrocytes that stop to multiply and are supplanted by bone and bone marrow. Therefore, development plate chondrocytes should be renewed ceaselessly and the immature microorganisms that fuel this procedure have for some time been looked for. Writing in Nature, Newton et al.1 and, already, Mizuhashi et al.2 have distinguished a sort of skeletal foundational microorganism in the resting zone of mouse bones that offers ascend to a wide range of development plate chondrocyte, just as a portion of the seemingly perpetual undifferentiated organisms of the bone-marrow stroma (non-blood-genealogy cells).
The two gatherings followed the relatives of individual chondrocytes by hereditarily altering the phones to express different fluorescent proteins. The creators recognized gradually separating cells in the resting zone that offer ascent to monoclonal (starting from a solitary cell) sections of multiplying and hypertrophic chondrocytes that range the whole development plate (Fig. 1).
The recently recognized cells communicated immature microorganism markers and could separate into numerous cell types. Besides, when named in adolescent mice, the cells kept on creating sections of chondrocytes into adulthood. Newton et al. additionally explored how the cells partition, and found that they are kept up as torpid cells, however every so often experience a hilter kilter cell division that produces one self-recharging cell and another cell that is inclined to separation. Together, these perceptions show the foundational microorganism character of the recently distinguished cells and bolster the speculation that uncommon, hilter kilter undifferentiated organism divisions refill the chondrocyte pool of the development plate, which is extended further by the transient multiplication of the undeveloped cells' little girl cells (level chondrocytes).
How different are the immature microorganisms recognized in the two investigations? Newton et al. named chondrocytes that express sort II collagen. Such cells are probably going to incorporate the number of inhabitants in cells communicating parathyroid hormone-related protein (PTHrP) that were followed by Mizuhashi and associates. Prominently, in spite of the fact that resting-zone undifferentiated cells obviously have a place with the chondrocyte heredity, Mizuhashi and partners found that they express a tantamount arrangement of immature microorganism markers and experience a development procedure like that of bone-marrow foundational microorganisms. The components that control the arrangement of separation of both undifferentiated organism types are, be that as it may, in any case to be deciphered.
Where do the resting-zone immature microorganisms originate from? Embryonic bone development, as postnatal bone development, is driven by the expansion of chondrocytes, trailed by hypertrophic separation and the substitution of chondrocytes by bone. The procedure results in a solidified bone shaft that is flanked via ligament at the two closures. Newton et al. marked embryonic chondrocytes and found that some form into resting-zone undifferentiated organisms. These trials additionally uncovered that, before birth, singular sections of multiplying and hypertrophic chondrocytes have a multiclonal root, as opposed to being gotten from a solitary, self-restoring undifferentiated organism. This perception infers that embryonic and postnatal bone development is composed in shockingly unique ways.
How do cells got from embryonic chondrocytes gain an undeveloped cell character? The two investigations demonstrate that the indication of self-reestablishing potential is connected to the age of optional hardening focuses (territories where bone tissue shapes) at the finishes of bones not long after birth. Newton et al. examined the mammalian focus of rapamycin complex 1 (mTORC1) pathway, which has been accounted for to control undifferentiated organism function3. They found that chondrocyte-explicit enactment of mTORC1 flagging prompts a move from topsy-turvy to symmetric foundational microorganism divisions, and thus to an expanded number of undifferentiated cells in the resting zone. These perceptions emphatically bolster a job for mTORC1 in controlling the self-reestablishment capability of resting-zone undifferentiated organisms.
The two gatherings additionally examined the job of the protein Indian hedgehog (Ihh), an individual from the Hedgehog group of development factors that is communicated in early-separated hypertrophic chondrocytes. Ihh has been appeared to prompt the statement of PTHrP in resting-zone chondrocytes, which thusly hinders the untimely inception of hypertrophy in multiplying cells4. Also, both Ihh and PTHrP initiate chondrocyte proliferation3.
Newton et al. also, Mizuhashi et al. give proof that the restraint of Hedgehog flagging decreases the length of chondrocyte segments. Newton and associates likewise watched expanded multiplication and the outflow of qualities focused by Hedgehog proteins in resting-zone cells after actuation of the Hedgehog pathway. These discoveries recommend that Hedgehog flagging has a job in controlling the undifferentiated organism character of resting-zone cells.
Be that as it may, given that Ihh manages PTHrP articulation straightforwardly, the watched changes in chondrocyte-segment length and cell expansion may likewise be a result of adjusted PTHrP levels. Besides, when Newton et al. repressed Hedgehog flagging and actuated the mTORC1 pathway at the same time, some foundational microorganisms moved from the resting zone into the multiplying zone without separating into level cells. Together, these perceptions bolster a job for Ihh in managing undifferentiated organism multiplication instead of immature microorganism character. Given that the connection among Ihh and PTHrP flagging is unpredictable, it will test recognize unmistakably between the jobs of Ihh as a controller of undifferentiated cell multiplication, PTHrP articulation and the enlistment and support of 'stemness'.
The model of how ligament is supplanted by bone has changed generously in the previous couple of years. Beforehand, hypertrophic chondrocytes were thought to kick the bucket and after that be supplanted by bone-shaping cells called osteoblasts. Be that as it may, later destiny mapping thinks about have demonstrated that a small amount of hypertrophic chondrocytes separate into bone-framing osteoblasts or seemingly perpetual undeveloped cells and ancestor cells of the bone-marrow stroma5– 7. Mizuhashi et al. presently exhibit that cells that are relatives of resting-zone undifferentiated cells add deep down marrow stroma. Subsequently, such undifferentiated organisms appear to pursue an irregular way of separation, changing from foundational microorganisms of the chondrocyte ancestry into separated chondrocytes, and after that into multilineage immature microorganisms of the bone-marrow stroma.
Future examinations ought to illuminate what number of the postnatal bone-marrow undifferentiated cells plummet from resting-zone immature microorganisms, and whether these postnatal cells vary practically from other bone-marrow cells. Given that bone-marrow-determined skeletal undifferentiated cells are required for bone turnover and crack fix all through an individual's life, translating the particular highlights of the chondrocyte-inferred populace will be of high clinical pertinence.
The recognizable proof of a development plate-explicit skeletal undeveloped cell is an essential advance towards understanding human skeletal development and related illnesses, however numerous inquiries remain. Follow-up studies need to figure out which components other than Hedgehog and mTORC1 flagging incite and keep up the undifferentiated organism character of these cells, which kind of embryonic chondrocyte advances into a resting-zone undeveloped cell, and how the acceptance of that procedure is connected to the arrangement of auxiliary solidification focuses.
Further examinations additionally need to illuminate how the separation of undeveloped cells in the resting zone is managed, and which parts of the chondrocyte-explicit extracellular lattice (the system of proteins and sugar particles that encompasses cells) are required to create an immature microorganism specialty. At last, given that some hypertrophic chondrocytes separate into osteoblasts and bone-marrow foundational microorganisms, while others die5– 7, it is enticing to solicit whether the destiny from hypertrophic cells is now controlled by the unmistakable subtypes of resting-zone undifferentiated organism from which they start.

 admin
admin 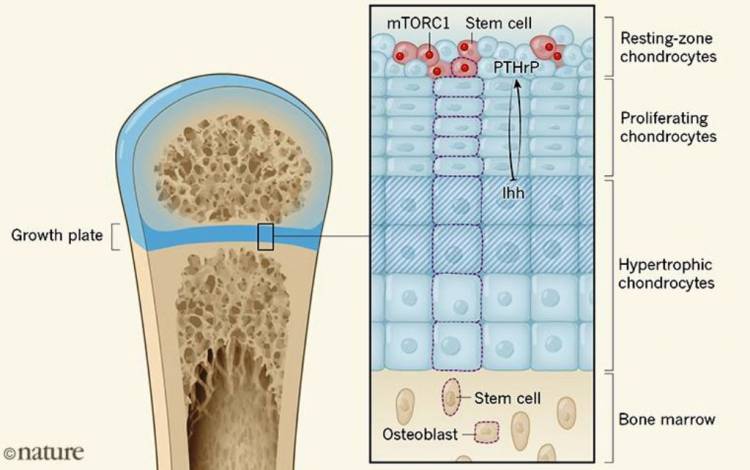


















Comments (0)
Facebook Comments (0)